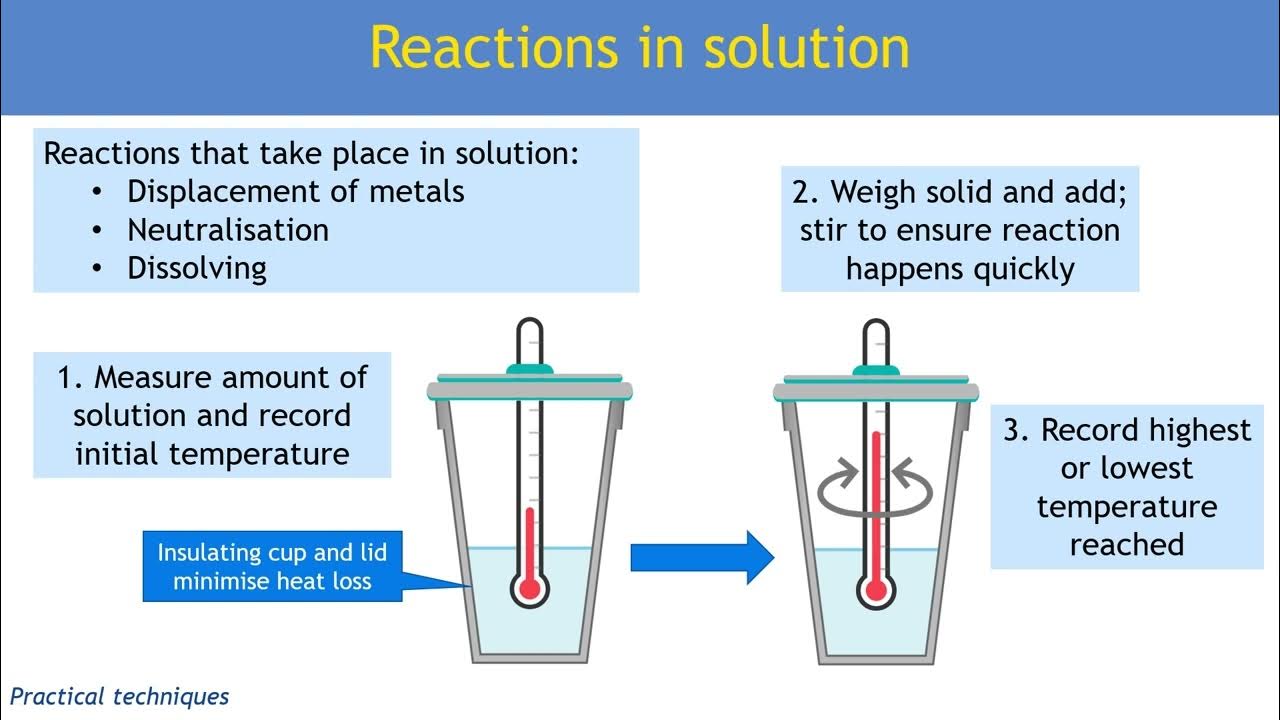
What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes . in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. two common enthalpy change measurements. what students need to know about measuring enthalpy changes? Know how to measure the enthalpy change accompanying the. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. often hess’s law cycles are used to measure the enthalpy change for a reaction that cannot be measured directly. generally, the measurement of enthalpy and internal energy is done by an experimental technique known as calorimetry. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.
from www.youtube.com
calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. often hess’s law cycles are used to measure the enthalpy change for a reaction that cannot be measured directly. what students need to know about measuring enthalpy changes? in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. Know how to measure the enthalpy change accompanying the. two common enthalpy change measurements. It uses devices called calorimeters, which measure the. generally, the measurement of enthalpy and internal energy is done by an experimental technique known as calorimetry.
Chemistry A Level Measuring Enthalpy Changes YouTube
What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes Know how to measure the enthalpy change accompanying the. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. It uses devices called calorimeters, which measure the. two common enthalpy change measurements. generally, the measurement of enthalpy and internal energy is done by an experimental technique known as calorimetry. often hess’s law cycles are used to measure the enthalpy change for a reaction that cannot be measured directly. Know how to measure the enthalpy change accompanying the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. what students need to know about measuring enthalpy changes?
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes two common enthalpy change measurements. what students need to know about measuring enthalpy changes? Know how to measure the enthalpy change accompanying the. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. often hess’s law cycles are used to measure the enthalpy change. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From www.youtube.com
Change in ENTHALPY Using Specific Heat at Constant Pressure in 3 What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes generally, the measurement of enthalpy and internal energy is done by an experimental technique known as calorimetry. two common enthalpy change measurements. It uses devices called calorimeters, which measure the. Know how to measure the enthalpy change accompanying the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. often hess’s law. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Know how to measure the enthalpy change accompanying the. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. often hess’s law cycles. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From www.pinterest.com
Laboratory Equipment Names in English • 7ESL Laboratory equipment What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes Know how to measure the enthalpy change accompanying the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. revision notes on 4.1.3 measurement of an enthalpy change for the aqa. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From mindcare.vn
9 different gauges Electronics & metrology Measuring & Test ENGINEERING What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. generally, the measurement of enthalpy and internal energy is done by an experimental technique known as calorimetry. Know how to measure the enthalpy change accompanying the. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From www.nagwa.com
Lesson Video Measuring Enthalpy Changes Nagwa What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. what students need to know about measuring enthalpy changes? It uses devices called calorimeters, which measure the. often hess’s law cycles are used to measure the enthalpy change for a reaction that. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From www.slideserve.com
PPT Chemistry The Molecular Science Moore, Stanitski and Jurs What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes two common enthalpy change measurements. It uses devices called calorimeters, which measure the. Know how to measure the enthalpy change accompanying the. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. generally, the measurement of enthalpy and internal energy is done. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From www.alamy.com
Measuring device used in the laboratory Stock Photo Alamy What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. what students need to know about measuring enthalpy changes? generally, the measurement of enthalpy and internal energy is done by an experimental technique known as calorimetry. in this explainer, we will. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From studylib.net
Grade 9 & 10 Scientific Equipment What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes often hess’s law cycles are used to measure the enthalpy change for a reaction that cannot be measured directly. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. Know how. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes often hess’s law cycles are used to measure the enthalpy change for a reaction that cannot be measured directly. Know how to measure the enthalpy change accompanying the. It uses devices called calorimeters, which measure the. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes what students need to know about measuring enthalpy changes? two common enthalpy change measurements. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. often hess’s. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. It uses devices called calorimeters, which measure the. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. what students. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From www.vrogue.co
Gcse A Level Chemistry Enthalpy Change Worksheets Les vrogue.co What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes Know how to measure the enthalpy change accompanying the. generally, the measurement of enthalpy and internal energy is done by an experimental technique known as calorimetry. two common enthalpy change measurements. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. what students need. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From www.dreamstime.com
Laboratory Measuring Equipment Stock Image Image of electrical What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes Know how to measure the enthalpy change accompanying the. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. in this explainer, we will learn how to perform calorimetry experiments and use the results to calculate the enthalpy change for a. often. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记4.1.4 Determining Enthalpy Change of What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes what students need to know about measuring enthalpy changes? It uses devices called calorimeters, which measure the. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Know how to measure the enthalpy change accompanying the. often hess’s law cycles are used. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From workshoprepaire1fr41k.z21.web.core.windows.net
Find Enthalpy Given Temperature And Pressure What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes what students need to know about measuring enthalpy changes? It uses devices called calorimeters, which measure the. generally, the measurement of enthalpy and internal energy is done by an experimental technique known as calorimetry. often hess’s law cycles are used to measure the enthalpy change for a reaction that cannot be measured directly. calorimetry is the. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From slideplayer.com
Lesson 5 Hess’ law and enthalpy cycles ppt download What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes what students need to know about measuring enthalpy changes? calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. generally, the measurement of enthalpy and internal energy is done by an experimental technique known as calorimetry. Know how to measure the enthalpy change accompanying the. revision notes on 4.1.3 measurement of an. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.
From printablelistquinta.z21.web.core.windows.net
Heat Of Reaction Chemistry What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes two common enthalpy change measurements. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. revision notes on 4.1.3 measurement of an enthalpy change for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Know how to measure the. What Is A Common Laboratory Measuring Device Used In Experiments That Measures Enthalpy Changes.